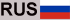Self-aware universe. How consciousness creates the material world. Chapter 3. Quantum physics and the demise of material realism
CHAPTER 3. QUANTUM PHYSICS AND THE DEATH OF MATERIAL REALISM
Almost a century ago, a number of experimental discoveries were made in physics that required a change in our worldview. What these experiments revealed were, in the words of philosopher Thomas Kuhn, anomalies that classical physics could not explain. These anomalies opened the way to a revolution in scientific thought.
Imagine that you are a physicist on the threshold of a new century. One of the anomalies you and your colleagues want to understand concerns how heated bodies emit radiation. As a Newtonian physicist, you believe that the universe is a classical machine made up of parts that behave according to the laws of Newtonian mechanics, which are almost all completely known. You believe that with all the information about the parts and the few remaining difficulties regarding the laws, you will be able to predict the future of the universe forever. However, these few remaining difficulties are unpleasant. You are not ready to answer questions concerning, for example, what is the law of radiation of heated bodies.
Imagine that while you are racking your brains over this question, your wife is sitting comfortably next to you in front of a burning fireplace.
You (mumbling): I just can’t understand it.
She : Pass me the nuts.
You (passing nuts ): I just can’t understand why we’re not sunbathing now.
She (laughing): Well, that would be nice. We might even have reason to use the fireplace in the summer.
You: You see, the theory says that the radiation from the fireplace should be as rich in ultraviolet light as sunlight. But what is it that makes sunlight, and not fireplace light, rich in these high frequencies? Why don’t we tan now by taking an ultraviolet bath?
She: Wait, please. For me to listen to this seriously, you’ll have to slow down a little and explain. What is frequency? What is ultraviolet?
You: Sorry. Frequency is the number of cycles per second. This is a measure of how quickly a wave oscillates. For light, this means color. White light is made up of light of different frequencies, or colors. Red is low frequency light and violet is high frequency light. If the frequency is even higher, then it is an invisible black color, which we call ultraviolet.
She: Okay, so both the light from burning wood and the light from the sun must contain a lot of ultraviolet radiation. Unfortunately, the sun obeys your theory, but burning wood does not. Perhaps there is something special about burning wood…
You: Actually, it’s even worse. All light sources, not just the sun or burning wood, should produce large amounts of ultraviolet radiation.
She: Ah, this is getting interesting. Ultraviolet inflation is omnipresent. But isn’t every inflation followed by a recession? Doesn’t the song say that everything that rises must fall? ( She starts humming without words.)
You (annoyed ): But how?
She ( holding out a bowl of nuts): Do you want some nuts, dear?
(The conversation ends.)
Planck makes his first quantum leap
At the end of the 19th century. Many physicists were disappointed until one of them broke the general trend – it was Max Planck from Germany. In 1900, Planck made a bold conceptual breakthrough when he declared that the old theory needed a quantum leap (he borrowed the word
quantum, meaning “quantity,” from Latin). The emission of light from hot objects—such as burning wood or the sun—is caused by electrons, tiny oscillating electrical charges. These electrons absorb energy from a heated environment, such as a fireplace, and then release it back as radiation. This part of the old physics was correct, but then classical physics predicted that the emitted radiation should be rich in ultraviolet, which our observations contradicted. Planck (very bravely) announced that the problem of emitting different amounts of ultraviolet light could be solved by assuming that electrons emit or absorb energy only in certain discrete portions, which he called “quanta” of energy.
To understand the meaning of an energy quantum, consider this analogy. Compare the case of a ball rolling down a staircase with the case of a ball rolling down an inclined plane (Fig. 1), which can occupy any position on the inclined plane, and its position can change by any amount. So this is a continuity model that represents the way we think in classical physics. By contrast, a ball on a staircase can only be on one step or another; its position (and its energy, which is associated with the position) is “quantized”.
Img. 1.
Quantum leap. On an inclined plane, the classical motion of a ball is continuous; on the quantum ladder, movement occurs in the form of discrete stages (quantum leaps)
You may object – what happens when the ball falls from one step to another? Doesn’t he occupy intermediate positions during his descent? This is where the unusualness of quantum theory comes into play. For a ball on a ladder, the answer must obviously be positive, but for the case of a quantum ball (atom or electron), Planck’s theory gives a negative answer. A quantum ball can never be found in any intermediate position between two steps; he is either on one or the other. This is quantum discontinuity.
So why can’t you get a tan from a wood burning fireplace? Imagine a pendulum in the wind Usually in such a situation the pendulum will swing, even if the wind is not very strong. Suppose, however, that the pendulum can absorb energy only in discrete portions of large magnitude. In other words, it is a quantum pendulum. What then? It is clear that unless the wind is capable of producing the required high increase in energy in one step, the pendulum will not move. Absorbing small amounts of energy will not allow it to accumulate enough energy to overcome the threshold. So it is with oscillating electrons in a fireplace. Small quantum jumps produce low-frequency radiation, but high-frequency radiation requires large quantum jumps. A large quantum jump must be caused by a large amount of energy in the environment surrounding the electron; The energy of wood burning in a fireplace is simply not strong enough to create the conditions to produce large amounts of blue light, let alone ultraviolet light. This is the reason why you can’t get a tan while sitting by the fireplace.
As far as we know, Planck was a fairly traditional scientist and was reluctant to make his ideas about energy quanta public. He even did his mathematics while standing, as was customary in Germany at that time. He didn’t particularly like the consequences of his innovative idea; however, it was becoming clear to the scientists who were to take the revolution much further that they were pointing to an entirely new way of understanding our physical reality.
Einstein’s photons and Bohr’s atom
One of these revolutionaries was Albert Einstein. At the time he published his first research paper on quantum theory, he was working as a clerk in the patent office in Zurich (1900). Questioning the then-popular idea of the wave nature of light, Einstein hypothesized that light exists in the idea of a quantum—a discrete bundle of energy—that we now call a photon. The higher the frequency of light, the more energy each beam has.
An even greater revolutionary was the Danish physicist Niels Bohr, who in 1913 used the idea of the quantum of light to formulate the hypothesis that the entire atomic world is full of quantum leaps. We have all been taught that the atom is like a miniature solar system, that electrons orbit the nucleus much like the planets orbit the sun. You may be interested to know that this model, proposed by the English physicist Ernest Rutherford, had a crucial flaw that Bohr’s work corrected.
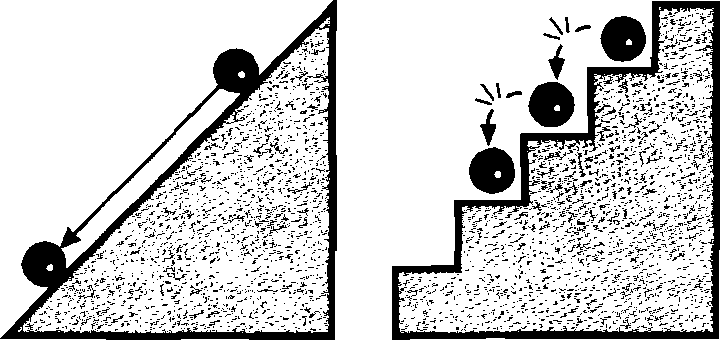
Imagine a swarm of orbiting satellites that are launched quite regularly from Earth using space rockets. These satellites do not exist forever. Due to collision with the earth’s atmosphere, they lose energy and slow down their movement. Their orbits narrow and they eventually fall to Earth (Fig. 2).
Img. 2.
The orbits of satellites revolving around the Earth are unstable. The orbits of electrons behave in the same way in the Rutherford model of the atom.
According to classical physics, the electrons surrounding the atomic nucleus would also lose energy due to the continuous emission of light and eventually fall into the nucleus. Therefore, the planetary model of the atom is unstable. However, Bohr (who supposedly saw the planetary system of the atom in a dream) created a stable model of the atom using the idea of a quantum leap.
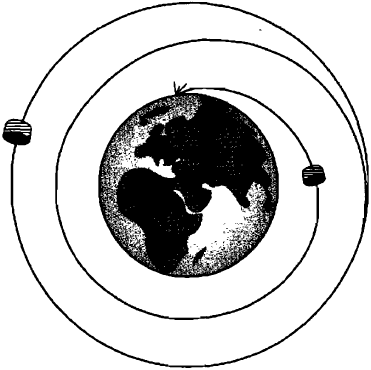
Suppose, Bohr said, that the orbits of electrons are discrete, like Planck’s energy quanta. The orbits can then be thought of as forming an energy ladder (Fig. 3). They are stationary – the amount of their energy remains unchanged. While in these quantized orbits, electrons do not emit light. An electron emits a quantum of light only when it jumps from a higher energy orbit to a lower orbit (from a higher energy rung of the ladder to a lower rung). Thus, if an electron is in the lowest energy orbit, it has no lower level to jump to. This base level configuration is stable and the electron has no chance of falling into the nucleus. All physicists greeted Bohr’s atomic model with a sigh of relief.
Img. 3.
Bohr’s orbit and quantum leap: a – quantized Bohr orbits. Atoms emit light as electrons jump from orbit to orbit; b – for quantum leaps along the energy ladder there is no need to pass through the intermediate space between steps
Bohr cut off the head of the Hydra of Instability, but another grew in its place. According to Bohr, an electron can never occupy any position between orbits; thus, when it makes a jump, it must somehow directly transfer to another orbit. This is not an orbital jump through space, but something radically new. Although it might be tempting to picture an electron’s jump as jumping from one rung of a ladder to another, the electron makes the jump without crossing the space between the rungs. Instead, it seems to disappear on one step, reappearing on another – without any continuous transition. Moreover, it is impossible to say where he is going to jump if there is more than one lower step between which he can choose. Only probabilistic predictions can be made.
Wave-particle duality
You may have noticed something strange about the quantum concept of light. To say that light exists in the form of quanta, photons, is to say that light consists of particles like grains of sand. However, such a statement largely contradicts the everyday experience that we gain when dealing with light.
Imagine, for example, looking at a distant street lamp through the fabric of a cloth umbrella. You won’t see a continuous stream of light passing through, as you would expect if the light was made up of tiny particles (put sand in a sieve and you’ll see what I mean). Instead, you’ll see a pattern of alternating dark and light borders, which is technically called an interference pattern. Light bends in and around the threads of fabric, creating a pattern that only waves can create. Thus, even our everyday experience shows that light behaves like a wave.
However, quantum theory insists that light also behaves as a beam of particles, or photons. Our eyes are such a wonderful instrument that we can observe for ourselves the quantum, grainy nature of light. The next time you part with a loved one at dusk, pay attention to how you see a retreating figure. Notice that the outline of the receding object appears fragmented. If the light energy bouncing off that object and hitting the optical receptors on your retina had wave-like continuity, then at least some light from every part of the object should always excite your optical receptors. You would always see the full image. (Admittedly, in low light, the contrast between dark and light would not be very clear, but this would not affect the clarity of the outlines.) However, what you see instead is not clear outlines at all, because the receptors in your eyes respond to individual photons. Dim light has fewer photons than bright light; so in this hypothetical twilight situation, only a few of your receptors will be stimulated at any given time—too few to detect the outline of a dimly lit figure. Therefore, the image you see will be fragmented.
Another question you may be wondering is: why can’t receptors store data indefinitely until the brain has collected enough information to put all the fragmented pictures together? Fortunately for quantum physicists, who are always desperate for everyday examples of quantum phenomena, optical receptors can only store information for fractions of a second. In dim light, at any given moment, not enough receptors in your eyes will be stimulated to create a complete image. The next time you say goodbye to the shadowy, retreating figure of a loved one at dusk, remember to think about the quantum nature of light; this will surely reduce the pain of your separation.
When light is considered as a wave, it is capable of being in two (or more places) at the same time – as is the case when it passes through the holes in the fabric of an umbrella, and forms a diffraction pattern; however, when we capture it on photographic film, it appears discretely, in separate specks, like a stream of particles. Thus light must be both a wave and a particle. Paradoxical, isn’t it? The case concerns one of the bastions of old physics: unambiguous description in natural language. Moreover, the very idea of objectivity is at stake: does the nature of light—what light is—depend on how we observe it?
And as if the paradoxes surrounding light weren’t challenging enough, another question inevitably arises: can a material object, such as an electron, be both a wave and a particle? Can he have a duality like the duality of light? The physicist who first posed this question and persistently gave a positive answer to it, which shocked all his colleagues, was the French aristocrat Louis Victor de Broglie.
Waves of matter
When de Broglie wrote his PhD thesis around 1924, he drew a parallel between the discreteness of the stationary orbits of the Bohr atom and the discreteness of sound waves produced by a guitar. The parallel turned out to be fruitful.
Imagine the movement of a sound wave in some medium (Fig. 4). The vertical displacement of the particles of the medium changes from zero to a maximum (ridge), back to zero, to a negative maximum (trough), again to zero, and so on with increasing distance. The maximum vertical displacement in one direction (from zero to a crest or trough) is called amplitude. Individual particles of the medium move back and forth relative to their resting position. However, a wave passing through a medium spreads. A wave is a propagating disturbance. The number of crests passing through a given point per second is called the wave frequency, and the distance from crest to crest is called the wavelength.
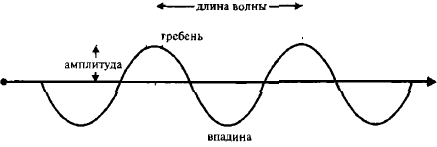
Img. 4.
Graphical representation of the wave
Plucking a guitar string sets it in motion, but the resulting vibrations are called stationary (standing waves) because they do not propagate beyond the string. At any given location on the string, the displacement of the string particles changes over time: waviness occurs, but the waves do not propagate in space (Fig. 5). The propagating waves that we hear are driven by standing waves of vibrating strings.
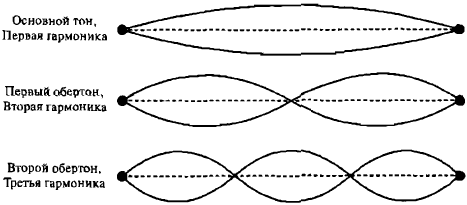
Img. 5.
The first few harmonics of a stationary, or standing, wave in a guitar string
A musical note on a guitar consists of a range of sounds—a spectrum of frequencies. De Broglie was interested in the fact that the standing waves of a guitar string create a discrete spectrum of frequencies called harmonics. The lowest frequency sound is called the first harmonic, which determines the tone we hear. Higher harmonics—the musical sounds that give a note its characteristic quality—have frequencies that are multiples of the first harmonic.
Stationarity is a property of waves in a confined space. Such waves can easily be produced in a cup of tea. De Broglie asked whether the electrons of an atom are localized (confined) waves? If so, do they form discrete stationary wave patterns? For example, perhaps the lowest atomic orbit is the one in which a single electron produces a stationary wave of the lowest frequency—the first harmonic—and higher orbits correspond to stationary electron waves of higher harmonics (Figure 6).
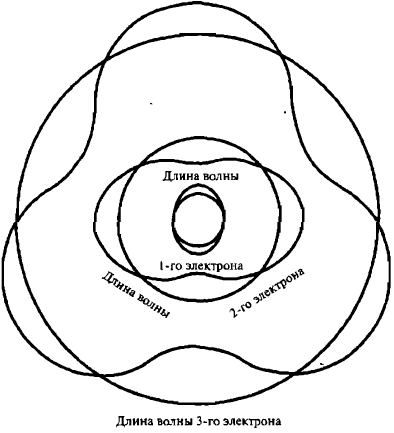
Img. 6.
De Broglie’s idea: couldn’t electrons be stationary waves in the limited space of an atom?
Of course, de Broglie used much more complex arguments to support his idea, but he still had difficulty getting his dissertation approved. Eventually it was sent to Einstein for review. Einstein, who first recognized the dual nature of light, had no difficulty in realizing that de Broglie might well be right: matter could well be as dual as light. De Broglie was awarded his degree when Einstein commented on his thesis: “It may look crazy, but it is actually logical.”
In science, experiment is always the final arbiter. The correctness of de Broglie’s idea about the wave nature of the electron was brilliantly demonstrated by an experiment in which a beam of electrons was passed through a crystal (a three-dimensional “umbrella” suitable for electron diffraction) and photographed. The result was a diffraction pattern (Fig. 7).

Img. 7.
Concentric diffraction rings show the wave nature of electrons
If matter is a wave, one physicist quipped to another at the end of a 1926 seminar on de Broglie waves, then there must be a wave equation that describes the wave of matter. The physicist who made this remark immediately forgot about it, but the one who heard it, Erwin Schrödinger, went on to discover the wave equation for matter, now known as the Schrödinger equation. It is the cornerstone that replaced Newton’s laws in the new physics. The Schrödinger equation is used to predict all the amazing qualities of submicroscopic objects found in our laboratory experiments. Werner Heisenberg discovered this same equation even earlier, but in a less clear mathematical form. The mathematical formalism that grew out of the work of Schrödinger and Heisenberg is called quantum mechanics.
The idea of matter waves proposed by de Broglie and Schrödinger gives rise to an amazing picture of the atom. It explains in simple terms the three most important properties of atoms: their stability, their identity with each other, and their ability to regenerate. I have already explained how stability arises – this was Bohr’s great contribution. The identity of atoms of a certain kind is simply a consequence of the identity of wave patterns in a limited space; the structure of stationary patterns is determined by the way in which the movement of electrons is limited, and not by their environment. The music of the atom, its wave pattern, remains the same no matter where it is – on Earth or in the Andromeda nebula. Moreover, a stationary pattern, depending only on the conditions of its limitation, has no trace of past history, no memory; it is restored again and again in the same form.
Waves of Probability
Electron waves are not like ordinary waves. Even in a diffraction experiment, individual electrons appear on a photographic plate as localized individual events; It is only by observing the pattern created by the entire beam of electrons that we discover evidence of their wave nature—the diffraction pattern. Electron waves are waves of probability, said physicist Max Born. They give us probabilities: for example, we are very likely to find a particle where the wave disturbances (or amplitudes) are large. If the probability of finding a particle is small, the amplitude of the wave will be weak. Imagine watching traffic from a helicopter hovering over the streets of Los Angeles. If cars were described by Schrödinger’s equation, we would say that the wave is strong in areas of traffic jams, and the wave is weak between traffic jams.
In addition, electron waves are usually represented as
wave packets . Using the concept of packets, we can make the wave amplitude large in certain regions of space, and small in all other places (Fig. 8). This is important because the wave must represent a localized particle. A wave packet is a packet of probability, and Born argued that for electron waves, the square of the wave amplitude—technically called the wave function—at some point in space gives us the probability of finding an electron at that point. This probability can be represented by a bell-shaped curve (Fig. 9).
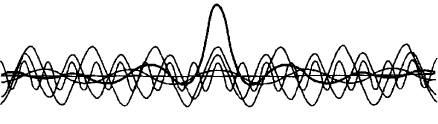
Img. 8.
The superposition of many simple waves forms a typical local wave packet (From P. W. Atkins’s book Quanta: A Handbook of Concepts, Oxford: Clairdon Press, 1974)

Img. 9.
Typical probability distribution
Heisenberg Uncertainty Principle
Probability breeds uncertainty. For an electron or any other quantum object, we can only talk about the probability of its being in such and such a place, or that its momentum (mass times velocity) is equal to such and such, but these probabilities form a distribution described by a bell-shaped curve. The probability will be maximum for some position value, and this will be the most likely location of the electron. However, there will be a whole range of positions in which there is a significant chance of finding an electron. The width of this region corresponds to the uncertainty of the electron position. The same arguments allow us to talk about the uncertainty of the electron momentum.
Based on similar considerations, Heisenberg mathematically proved that the product of the uncertainties of the electron’s position and momentum is greater than or equal to a certain small number called Planck’s constant. This number, originally discovered by Planck, sets the quantitative scale at which quantum effects become usably large. If Planck’s constant were not so small, the effects of quantum uncertainty would even invade our everyday macroscopic reality.
In classical physics, any movement is determined by the forces that control it. Once we know the initial conditions (the position and momentum of an object at some initial time), we can calculate its exact trajectory using Newton’s equations of motion. Therefore, classical physics leads to the philosophy of determinism – the idea of the possibility of completely predicting the movement of all material objects.
The principle of uncertainty undermines the philosophy of determinism. According to the uncertainty principle, we cannot accurately determine the position and speed (or momentum) of an electron at the same time; any attempt to accurately measure one makes knowledge of the other uncertain. Therefore, the initial conditions for calculating a particle’s trajectory can never be precisely determined, and the concept of a well-defined particle trajectory becomes unusable.
For the same reason, Bohr’s orbits do not provide a strict description of the location of the electron: the position of the actual orbits is uncertain. We really cannot say that an electron located at one energy level or another is located at such and such a distance from the nucleus.
Dubious fantasies
Let’s consider several fantastic scenarios, the authors of which did not realize the importance of the uncertainty principle or forgot about it.
In the science fiction book Fantastic Voyage and the movie based on it, objects were made miniature by compaction. Have you ever wondered if it is possible to compress atoms? After all, they are mostly made up of empty space. Is this possible? Decide for yourself based on the uncertainty principle. The size of an atom gives a rough idea of the degree of uncertainty in the position of its electrons. Densifying an atom will place its electrons in a smaller volume of space, thereby reducing the uncertainty of their position; but the uncertainty of their momentum must increase. Increasing the uncertainty of the electron’s momentum means increasing its speed. Thus, as a result of compaction, the speed of electrons increases and they are more able to leave the atom.
In another example of science fiction, Captain Kirk (from the classic television series Star Trek) gives the command: launch! A button is pressed on the dashboard and whoops, the people standing on the platform disappear, appearing at a destination that is supposed to be an unexplored planet but looks a lot like a Hollywood set. In one of his novels based on the Star Trek series, James Blish tried to characterize this process as a quantum leap. Just as an electron jumps from one atomic orbit to another without crossing the space in between, so would the crew of the starship Enterprise. You can see what the problem is here. When and where the electron will make the leap is not subject to the law of causality and is unpredictable due to the laws of probability and uncertainty of the quantum leap. Such a quantum transport would force the heroes of the Enterprise, at least sometimes, to wait a very long time to get somewhere.
Quantum fantasies may be fun, but the ultimate goal of new physics and this book is serious. It is to help us deal with our daily reality.
Wave-particle duality and quantum measurement
The preceding background information helps explain a couple of puzzling questions. Does the quantum picture of an electron moving in waves around a nucleus imply that the electron’s charge and mass are spread throughout the atom? And does the fact that a free electron propagates as a wave should propagate according to Schrödinger’s theory mean that its charge is now spread throughout space? In other words, how can the wave pattern of an electron be reconciled with the fact that it has the properties of a localized particle? The answers to these questions are quite complex.
It would seem that at least wave packets make it possible to confine an electron to a small space. Alas, everything is not so simple. A wave packet that satisfies the Schrödinger equation at a given time must spread over time.
At some initial moment we can localize the electron to a tiny point, but within seconds the wave packet of the electron will spread throughout the entire city. Although initially the probability of finding an electron in a tiny spot is overwhelmingly high, after just a few seconds the probability of an electron appearing anywhere in the city becomes significant. And if we wait long enough, the electron could appear anywhere in the entire country, or even the entire universe.
It is this propagation of the wave packet that contributes to the ongoing jokes about quantum predestination among cognoscenti. For example, take this quantum mechanical way of materializing a Christmas turkey: prepare the oven and wait – there is a non-zero probability that a turkey from a nearby store will materialize in your oven.
Unfortunately for the turkey lover, for objects as massive as the turkey, propagation is extremely slow. To materialize even a small piece of turkey in this way might have to wait for the entire existence of the universe.
What about the electron? How to reconcile the propagation of an electron wave packet throughout the city with the picture of a localized particle? The answer is that we must take the act of observation into account in our calculations.
If we want to measure the charge on an electron, we must trap it in something like a cloud of vapor in a condensation chamber. As a result of this measurement, we must assume that the electron wave collapses, so that we are now able to see the path of the electron through the vapor cloud (Fig. 10). According to Heisenberg, “the path of an electron only comes into existence when we observe it.” When we make a measurement, we always find an electron localized as a particle. We can say that our measurement reduces the electron wave to the particle state.
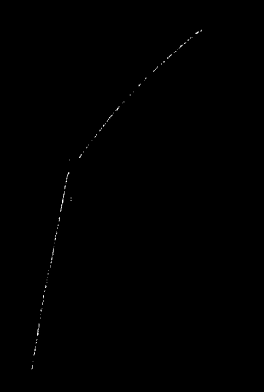
Img. 10.
Electron track in a vapor cloud
When Schrödinger proposed his wave equation, he and others thought that they might have freed physics from quantum jumps—from discontinuity—since wave motion is continuous. However, the corpuscular nature of quantum objects had to be reconciled with their wave nature. Therefore, wave packets were proposed. Finally, with the recognition of the propagation of the wave packet and the realization that it is the measurement that must cause the instantaneous collapse of the packet dimensions, we see that the collapse must be intermittent (continuous collapse would take time).
It seems as if there can be no quantum mechanics without quantum leaps. Schrödinger once visited Bohr in Copenhagen, where he spent days protesting against quantum leaps. It is said that he finally gave up, exclaiming in irritation: “If I had known that it was necessary to recognize this damned quantum leap, I would never have gotten involved with quantum mechanics.”
Let’s go back to the atom: if we measure the position of an electron in an atomic stationary state, we again collapse its cloud of probability, finding it in a certain position, and not smeared all over the place. By making a large number of measurements in search of an electron, we will more often find it in places where the probability of finding it is high, in accordance with the prediction of the Schrödinger equation. Indeed, if after a large number of measurements we plot the measured positions, it will look exactly like the blurred orbital distribution that the solution of the Schrödinger equation gives (Fig. 11).
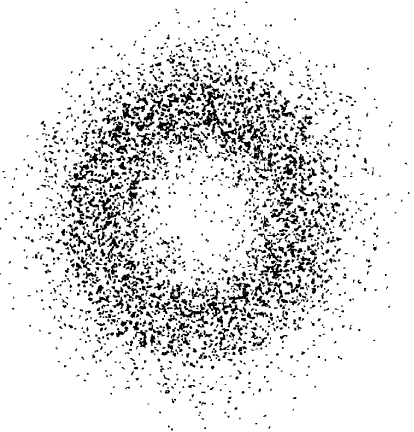
Img. 11.
Results of multiple measurements of the position of the electron in the hydrogen atom in the lowest orbit. It is obvious that the electron wave usually collapses where the predicted probability of finding it is high, resulting in a fuzzy orbit
What does a flying electron look like from this point of view? When we make an initial observation of any propagating submicroscopic object, we find it localized as a particle in a tiny wave packet. However, after observation, the packet dissipates, and the dispersion of the packet represents the cloud of our uncertainty about the packet. If we observe again, the packet is localized again, but between our observations it always dissipates.
According to physicist-philosopher Henry Margenau, seeing electrons is like seeing fireflies on a summer evening. You may see a flash here, and a flicker of light there, but have no idea where the firefly is between your sightings. You cannot determine its trajectory with any certainty. Even for such a macroscopic object as the moon, quantum mechanics predicts essentially the same picture – the only difference is that the scattering of the wave packet is immeasurably small (but non-zero between observations).
Now we come to the heart of the matter. Whenever we measure a quantum object, it appears in one place as a particle. The probability distribution simply identifies the place (or places) where it is likely to be found when we measure it – nothing more. When we don’t measure it, a quantum object scatters and exists at the same time in more than one place, just like a wave or a cloud – no less.
Quantum physics offers a new and exciting worldview that challenges old concepts such as deterministic trajectories and causal continuity. If initial conditions do not forever determine the movement of an object, if every moment we observe becomes a new beginning, then at a fundamental level the world is creative.
There was a Cossack who saw how almost every day, at approximately the same time, a rabbi crossed the city square. One day, out of curiosity, he asked, “Where are you going, Rabbi?”
The rabbi answered: “I don’t know for sure.”
“You pass this road every day at this time. Of course you know where you are going.”
When the rabbi insisted that he did not know this, the Cossack became angry, then became suspicious and finally took the rabbi to prison. Just as he was locking the cell door, the rabbi looked at him and said softly, “See, I didn’t know.”
Before the Cossack stopped him, the rabbi knew where he was going, but after that he no longer knew. Stopping (we can call it measuring) opened up new possibilities. This is the meaning of quantum mechanics. The world is not determined once and for all by initial conditions. Every measurement event is potentially creative and can open up new possibilities.
The principle of complementarity
A new way of understanding the paradox of wave-particle duality was proposed by Bohr. According to him, the wave and particle nature of the electron are not dual, but simply polar opposite qualities. These are complementary qualities that are revealed to us in complementary experiments. When we take the diffraction pattern of an electron, we discover its wave nature; when we trace it in a cloud of vapor, we see its corpuscular nature. Electrons are neither waves nor particles. They can be called “wave particles” because their true nature transcends both descriptions. This is the principle of complementarity.
Since contemplating the fact that the same quantum object has such seemingly contradictory properties as wave and particle can be dangerous for the human psyche, nature has provided a shock absorber. Bohr’s principle of complementarity assures us that although quantum objects have both wave and particle properties, we can, in any experimental setting, measure only one aspect of a wave particle at any given time. We choose which aspect of the wave particle we want to see by choosing the appropriate experimental setting.
Principle of correspondence
Having understood the revolutionary ideas of the new physics, it would be completely wrong to think that Newton’s physics was completely wrong. The old physics lives on in the realm of most (but not all) gross matter as a special case of the new physics. An important feature of science is that when a new order replaces an old one, it usually expands the scope of its application. In the old region, the mathematical equations of old physics remain valid (confirmed by experimental data). Therefore, in the realm of classical physics, the conclusions of quantum physics about the motion of objects correspond clearly to those made using Newtonian mathematics under the assumption that the bodies with which we are dealing are classical. Bohr formulated this principle of correspondence. The relationship between classical and quantum physics is in some ways like a visual illusion. “My wife and my mother-in-law” (Fig. 12). What do you see in this picture? First you see either the wife or the mother-in-law. I always see my wife first. In fact, it may take you a while to spot the second image in the drawing. If you look closely at it, suddenly a second image appears. The wife’s chin becomes the mother-in-law’s nose, her neck becomes the chin of an older woman, and so on. You may be wondering – what’s going on? The lines of the drawing remain the same, but suddenly a new way of perceiving the painting becomes possible for you. Very soon you discover that you can easily move from the old picture to the new one and back again. At any moment you still see only one of the two images, but your consciousness has expanded so that you are aware of their duality. In this expanded state of awareness, the strangeness of quantum physics begins to become clear. It even becomes exciting. To paraphrase Hamlet’s words addressed to Horatio, we can say that there are many things in heaven and on earth that classical physics never dreamed of.

Img. 12.
My wife and my mother-in-law
Quantum mechanics gives us a broader perspective, a new context that expands our perception into a new area. We can see nature as separate forms – waves or particles – or we can detect complementarity: the idea that the same thing has both wave and particle properties.
Copenhagen interpretation
According to the so-called Copenhagen interpretation of quantum mechanics, developed by Bohr, Heisenberg and Born, we calculate quantum objects as waves and interpret the waves in a probabilistic manner. We define their attributes, such as position and momentum, somewhat vaguely and understand them in terms of the principle of complementarity. In addition, lack of continuity and quantum jumps—for example, the collapse of an expanding wave packet upon observation—are considered fundamental aspects of the behavior of a quantum object. Another aspect of quantum mechanics is inseparability. Talking about a quantum object without talking about how we observe it makes no sense, since one is inseparable from the other. Finally, for massive macroobjects, the predictions of quantum mechanics coincide with the predictions of classical physics. This prohibits the manifestation of such quantum effects as probability and discontinuity in the macroscopic sphere of nature, which we observe directly with the help of our senses. Classical correspondence masks quantum reality.
Overcoming material realism
The principles of quantum theory make it possible to abandon the unfounded assumptions of material realism.
Assumption 1: Strict objectivity . The basic assumption of materialism is that there is a material universe independent of us. This assumption has some obvious operational validity and is often considered necessary for the meaningful pursuit of science. Is this assumption really justified? Quantum physics shows that we choose which aspect – wave or particle – a quantum object will demonstrate in a given situation. Moreover, our observation collapses the quantum wave packet into a localized particle. Subjects and objects are inseparably linked together. If this is so, how can one adhere to the assumption of strict objectivity?
Assumption 2: Causal determinism. Another assumption of classical science that underpins material realism is that the world is fundamentally deterministic: we only need to know the forces acting on each object and the initial conditions (the initial speed and position of the object). However, the principle of quantum uncertainty says that we can never simultaneously determine both the speed and position of an object with absolute accuracy. Our knowledge of the initial conditions will always contain error, and strict determinism is unacceptable. The very idea of causality is equally suspect. Since the behavior of quantum objects is probabilistic, a strict cause-and-effect description of the behavior of a single object is impossible. Instead, when we talk about large groups of particles, we have statistical cause and statistical effect.
Assumption 3: Locality. The assumption of locality—that all interactions between material objects are mediated by local signals—is crucial to the materialist view that objects exist essentially separately and independently of each other. However, if the waves travel over vast distances and then suddenly collapse when we make a measurement, then the influence of our measurement is not transmitted locally. Thus, locality is excluded. This is another death blow to material realism.
Assumptions 4 and 5. Materialism and epiphenomenalism. Materialism maintains that subjective mental phenomena are merely epiphenomena of matter and can be completely reduced to the material brain. However, according to the principle of complementarity and the idea of subject-object confusion, to understand the behavior of quantum objects, we seem to need to consider consciousness – our ability to make choices. Moreover, it seems absurd that an epiphenomenon of matter can affect matter: if consciousness is an epiphenomenon, then how can it “collapse” a dispersed wave of a quantum object into a localized particle when performing a quantum measurement?
Despite the principle of correspondence, the new paradigm of physics – quantum physics – contradicts the data of material realism. There is no way around this conclusion. We cannot say, invoking the correspondence principle, that classical physics is valid for macro objects for all practical purposes, and that since we live in the macro world, we will assume that quantum strangeness is limited to the submicroscopic sphere of nature. On the contrary, strangeness haunts us at the macro level. If we divide the world into the realms of classical and quantum physics, insoluble quantum paradoxes arise.
In India, people came up with a clever way to catch monkeys using a container of nuts. The monkey reaches into the container, grabbing a handful of nuts. Alas, having clenched the food in her fist, she can no longer pull her hand out – the neck of the vessel is too narrow. The trap works because the monkey’s greed prevents it from releasing the nuts. The axioms of material realism are materialism, determinism, locality, etc. – served us well in the past, when our knowledge was more limited than it is today, but now they have become a trap for us. We may have to give up the nuts of certainty to embrace the freedom that lies beyond the material arena.
If material realism cannot be an adequate philosophy for physics, then what philosophy can deal with all the strangeness of quantum behavior? This is the philosophy of monistic idealism that underlies all religions of the world.
Traditionally, only religions and the humanities recognize the value of human life beyond physical survival – a value derived from our love of beauty; our creative abilities in art, music and thought; and our spirituality in the intuition of unity. The natural sciences, locked within the framework of classical physics and its philosophical baggage of material realism, were a seducer of skepticism. Now the new physics is in dire need of a new, liberating philosophy suitable to the current level of our knowledge. If monistic idealism is the way to go, then the sciences and humanities, along with religions, will be able to go hand in hand in the search for all human truth for the first time since Descartes.
The book “The Self-Aware Universe. How consciousness creates the material world.” Amit Goswami
Contents
PREFACE
PART I. The Union of Science and Spirituality
CHAPTER 1. THE CHAPTER AND THE BRIDGE
CHAPTER 2. OLD PHYSICS AND ITS PHILOSOPHICAL HERITAGE
CHAPTER 3. QUANTUM PHYSICS AND THE DEATH OF MATERIAL REALISM
CHAPTER 4. THE PHILOSOPHY OF MONISTIC IDEALISM
PART II. IDEALISM AND THE RESOLUTION OF QUANTUM PARADOXES
CHAPTER 5. OBJECTS IN TWO PLACES AT THE SAME TIME AND EFFECTS THAT PRECEDE THEIR CAUSES
CHAPTER 6. THE NINE LIVES OF SCHRODINGER’S CAT
CHAPTER 7. I CHOOSE WITH THEREFORE, I AM
CHAPTER 8. THE EINSTEIN-PODOLSKY-ROSEN PARADOX
CHAPTER 9. RECONCILIATION OF REALISM AND IDEALISM
PART III. SELF-REFERENCE: HOW ONE BECOMES MANY
CHAPTER 10. EXPLORING THE MIND-BODY PROBLEM
CHAPTER 11. IN SEARCH OF THE QUANTUM MIND
CHAPTER 12. PARADOXES AND COMPLEX HIERARCHIES
CHAPTER 13. “I” OF CONSCIOUSNESS
CHAPTER 14. UNIFICATION OF PSYCHOLOGIES
PART IV . RETURN OF CHARM
CHAPTER 15. WAR AND PEACE
CHAPTER 16. EXTERNAL AND INTERNAL CREATIVITY
CHAPTER 17. THE AWAKENING OF BUDDHA
CHAPTER 18. IDEALISMAL THEORY OF ETHICS
CHAPTER 19. SPIRITUAL JOY
GLOBAR OF TERMS